
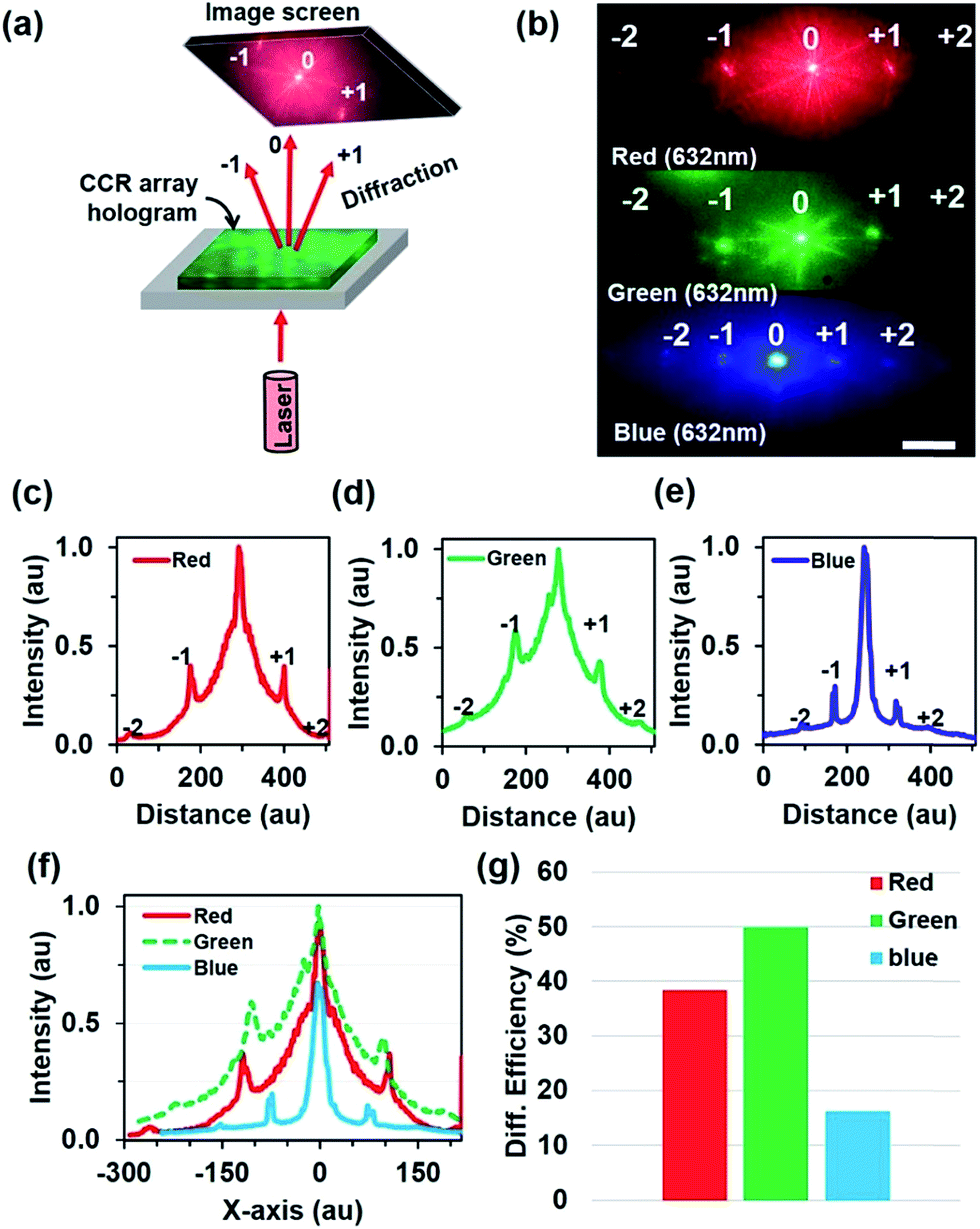
Salts of amoxapine with different acids were successfully developed, and their crystal structure was determined. Solubility of salts was determined in water by the shake-flask method at 37☌ using UV−vis spectroscopy. Salts were characterized by Fourier transform infrared spectroscopy, differential scanning calorimetry, and powder X-ray diffraction. Crystal structures of API salts were determined by single-crystal X-ray diffraction. Single crystals of cocrystals were grown by the solvent evaporation method in water, ethanol, and methanol. Amoxapine has very low solubility in water, so it was cocrystallized with natural acids in a 1:1 ratio in appropriate solvents by the solvent-drop grinding method. Amoxapine is a benzox-azepine derivative and exhibits antidepressant properties. The objective of pharmaceutical cocrystalliza-tion is to create crystalline analogues that have vastly different properties, such as solubility, melting point, stability, and bioavailability from that observed in the pure active pharmaceutical ingredients (APIs). Two guided inquiry research type assignments are herein discussed that expose students to crystallography and enhance literature review/analysis skills that allows them to become better undergraduate researchers.
Thus, the aim of this article is to touch upon the attempts to introduce the topic of crystallography to undergraduates of the inorganic chemistry laboratory at Georgia College and State University (GCSU). While accessibility to high-tech instrumentation is indeed an obstacle, there are educational sources that are quite accessible for undergraduate students, merely requiring a working computer to make use of them. State of the art X-ray diffraction instruments typically reach the ~$500,000 range and are rarely available to primarily undergraduate institutions. Several attempts have been made to introduce crystallography topics at the undergraduate level, but the one present obstacle is the availability of instrumentation for undergraduates to run experiments in. This boost in creep resistance is consistent with the enhanced aging response (higher Orowan stress).Chemical crystallography is considered one of the essential characterization techniques of inorganic chemistry since it is a critical tool in the structural determination of molecules small and large. At 300 ☌, creep threshold stresses are observed in both alloys in the peak-aged state, which increases from ∼30 MPa in the Sn-free alloy to ∼52 MPa in the Sn-modified alloy. Significant Sn segregation at the semi-coherent interfaces of the α-precipitates in the peak-aged Sn-modified alloy is observed via APT, which promotes homogeneous nucleation of the I/α-precipitates at aging temperatures > 400 ☌. High-resolution transmission electron microscopy analyses demonstrate that these Mn-Si-rich nanoprecipitates exhibit icosahedral quasicrystal ordering (I-phase), which transform into the cubic-approximant α-phase upon peak aging. Atom-probe tomography analyses reveal that the enhanced dispersion of the α-precipitates is related primarily to the formation of Sn-rich nanoprecipitates at intermediate temperatures, which act as nucleation sites for Mn-Si-rich nanoprecipitates. ∼10 19–20 m − 3) in the Sn-modified alloy. 100–500 nm) and their number density is greater (∼10 21 vs. Scanning electron microscopy and synchrotron x-ray diffraction analyses demonstrate that, while the structure of the α-Al(Mn,Fe)Si precipitates formed in the peak-aged alloys is identical, their mean radius is smaller (R ∼ 25 vs.
Isochronal aging experiments reveal that Sn inoculation results in a pronounced age-hardening response: a hardening increment of 125 MPa is achieved at peak-aging (475 ☌), which is five times greater than that of a Sn-free alloy. Precipitation-strengthening at ambient and high temperatures is examined in Al-0.5Mn-0.3Si (at.%) alloys with and without 0.02 at.% Sn micro-additions.


 0 kommentar(er)
0 kommentar(er)
